physical properties of air
air molecules
- nitrogen (N2) - 78.1%
- oxygen (O2)- 20.9%
- argon (Ar)- .9%
- carbon dioxide (CO2)- trace
- water vapor (H2O) - approx 1%
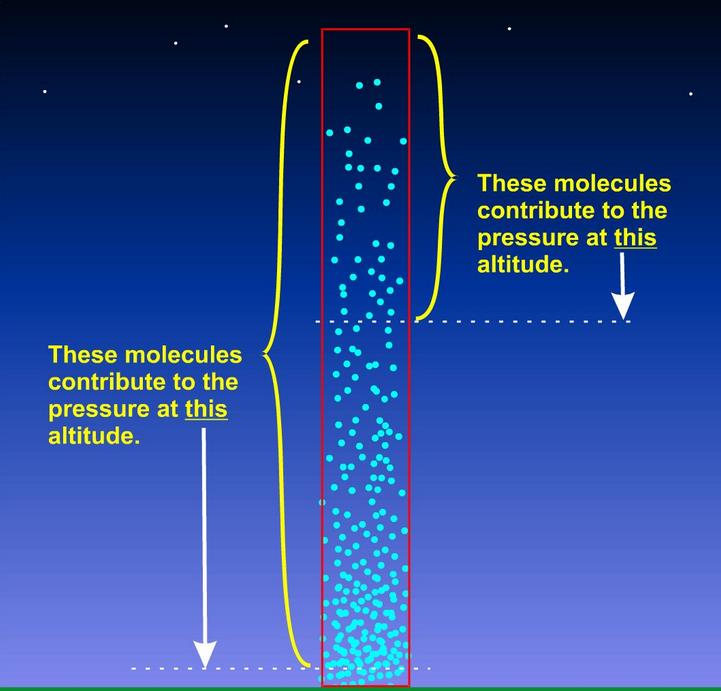
air pressure
- force exerted on an object at a certain elevation by its surrounding air molecules
- air molecules at a certain level are compressed by the weight of the air molecules above itself
- air molecules travel at an average speed of 1160 miles per hour
- this speed directly correlates with the speed of sound
- 1 cubic foot of air at sea level contains 7.6 x 10**23 molecules
air pressure by altitude
- sea level - 14.7 psi
- 5,000 feet - 12.2 psi
- 10,000 feet - 10.1 psi
- 18,000 feet - 7.3 psi
- Mount Everest -
- 50,000 feet - 1.6 psi
mean free path
- defined as the average distance any air molecule travels before colliding with another molecule
- mean free path at sea level = .3 /(10**6) feet
- this very short distance bounds how air molecules might react with moving objects
- movement of air from a high pressure region to a low pressure region
- at a microscopic level the air molecules are still jittering about as they collide with each other
- differing pressure regions are primarily caused on earth by:
- differential heating between the equator and the poles
- causes air to move from the equator to the pole
- Coriolis effect of the earth rotating on its axis
- causes air to move from west to east
- in northern hemisphere at the 45th parallel, the combination of the above two effects leads to wind on average to blow from the southwest
- local effects
- heating and cooling at shorelines of oceans and lakes
- mountain valleys and large hills
temperature
- an increase in air temperature causes an increase in the average air molecule speed
- as the molecular speed increases, the volume expands in the direction of decreasing pressure which is upwards
- the expansion causes a corresponding wind vector towards the low pressure area